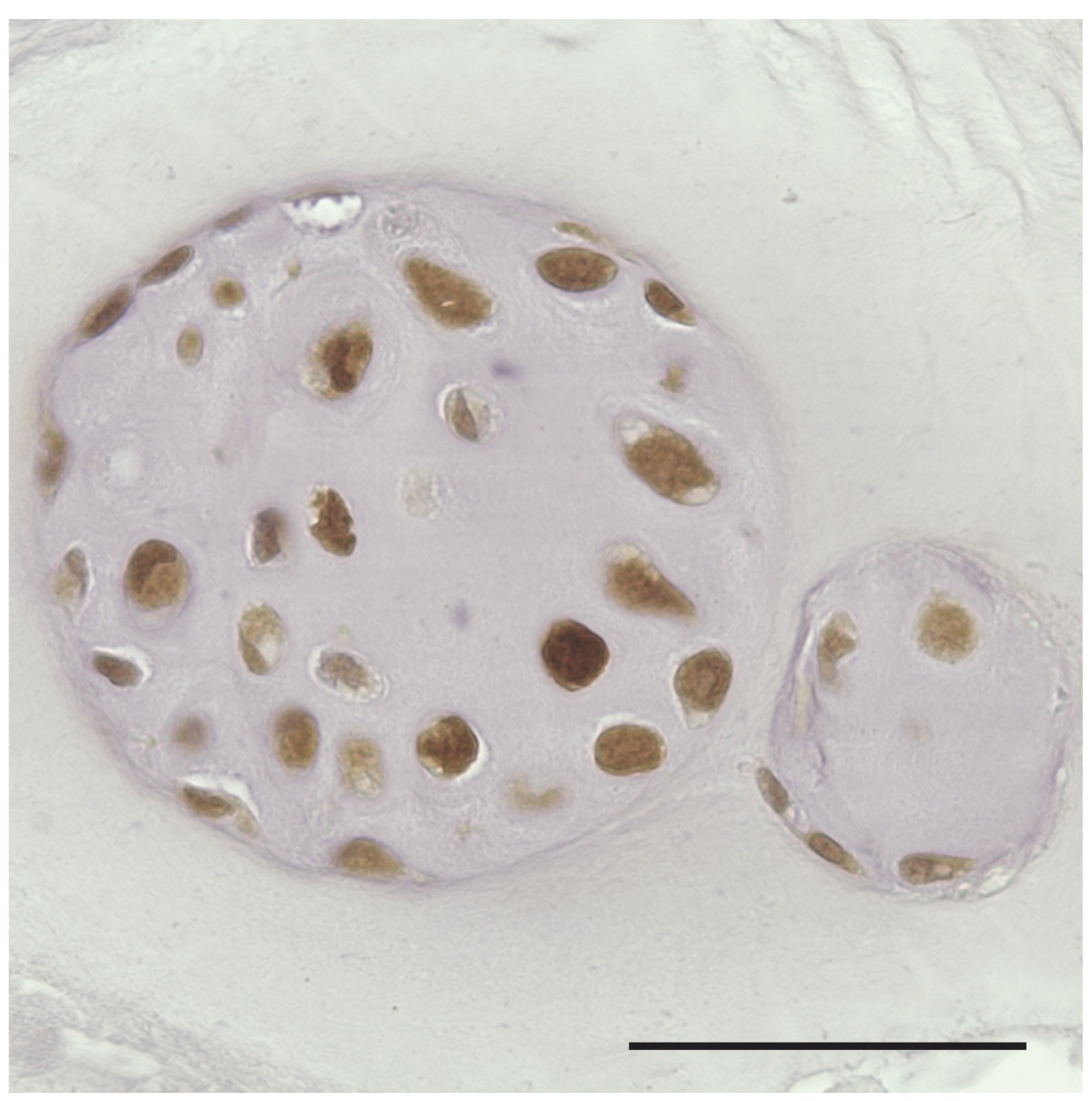
Coding of biological information is commonly attributed to nucleic acids and proteins. In terms of coding capacity, however, glycans surpass these two classes of macromolecules. Recent research has unraveled that the abundance of glycan-related information is translated into biological functions by the interplay with specific receptors (lectins).
At the KCLOB, we are among the first research groups to comprehensively investigate the biological relevance of glycan-lectin interactions for the onset and progression of degenerative joint diseases. Using an interdisciplinary approach by strategically combining glycan/lectin analysis on structural and functional levels, research emphasis is given to providing new insights to tissue development and degeneration, with perspectives for innovative medical applications.
For further information please contact Assoc.Prof. Mag. Dr. Stefan Tögel and see our publications.
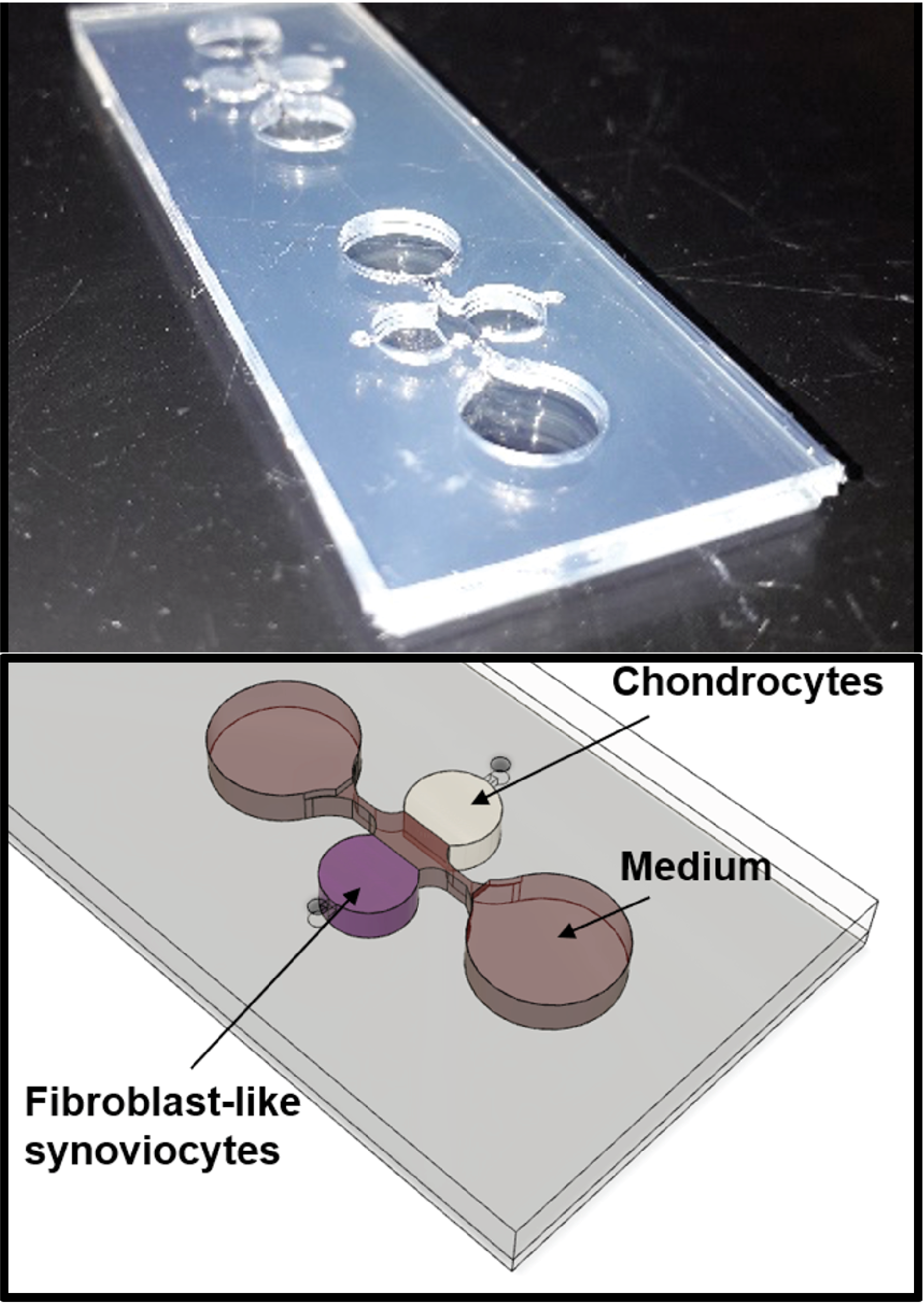
For over two decades, organs-on-a-chip have proven useful for recapitulating human physiology in health and disease. These so-called microphysiological systems are ideally suited to (i) reduce animal testing by generating human organ models, (ii) facilitate drug development and (ii) investigate personalized medicine aspects by integrating patient-derived primary cells.
At the KCLOB, we want to use organs-on-a-chip as three-dimensional models of the human joint to recapitulate onset and progression of degradative (enzymatic cartilage destruction) and inflammatory processes (cartilage and joint capsule inflammation), ranging from molecular pathways up to cellular and tissue-level architecture.
We believe that a systematic in vitro investigation of disease factors and co-factors that mediate OA using three-dimensional human joints-on-a-chip may be key in identifying basic biological pathways that govern the onset and progression of osteoarthritis.
For further information please contact Priv.Doz. Dr. Mario Rothbauer, see our publications, or visit the project website (under construction)..
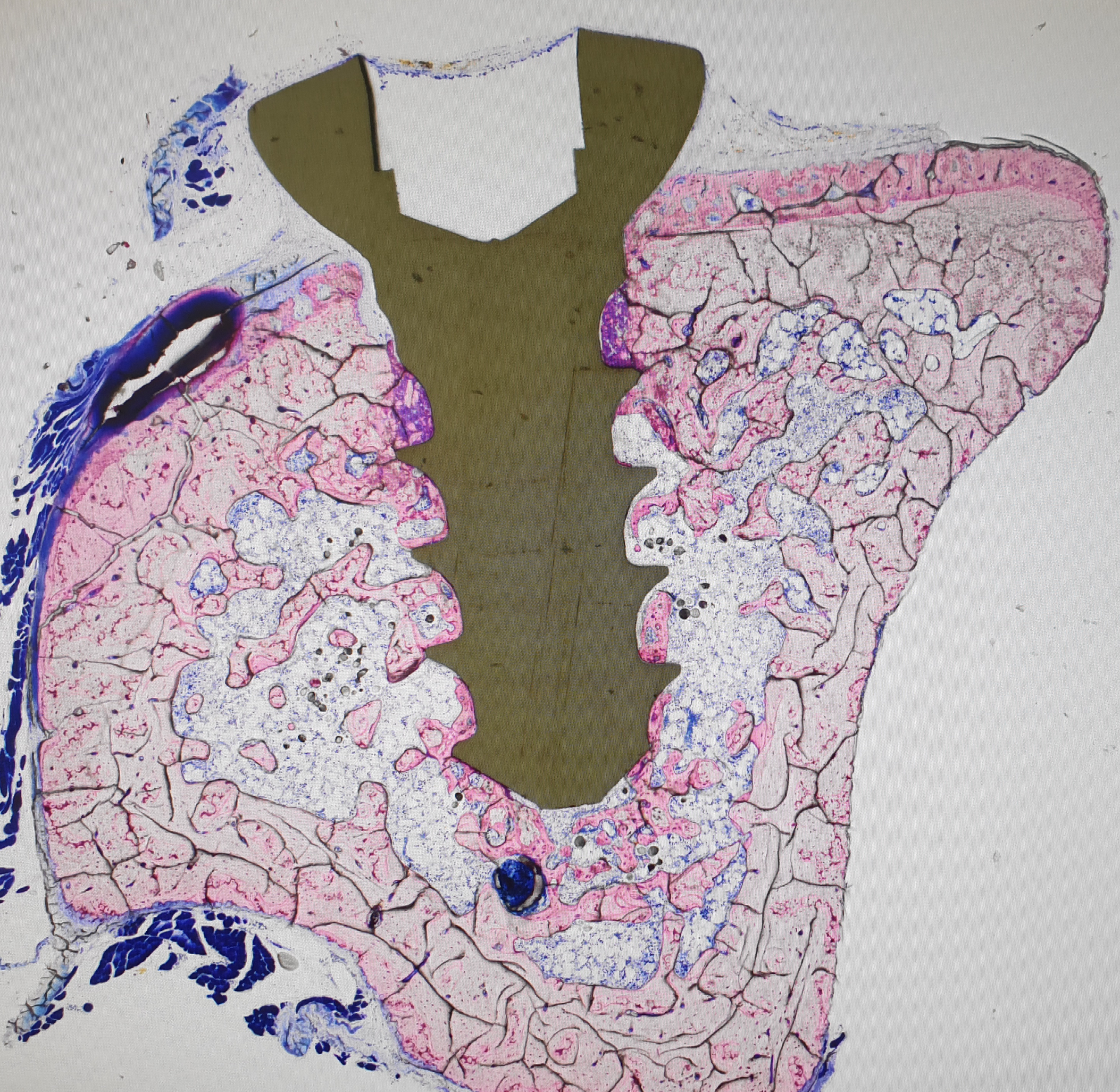
Aseptic implant loosening (AIL), a state where implants lose their attachment to the bone under non-infectious conditions, constitutes the most frequent cause for knee and hip revision arthroplasty. AIL is a result of mechanically induced osteolysis, which in turn occurs due to a lack of osseointegration, the biomechanical conjunction between implant and bone.
At the KCLOB, we strive to improve the bone-implant contact through a combination of in vitro and in vivo fundamental research. To this end, we investigate mesenchymal stem/stromal cells and their potential to differentiate into osteoblasts by employing various growth factors, mechanical stimulation and cultivation on titanium discs simulating the implant surface.
Additionally, we use animal models of postmenopausal osteoporosis to study how to enhance the biomechanical interface of titanium implants and living bone tissue. We anticipate that our research will provide valuable insights into how osseointegration of orthopedic implants is regulated and how this process can be improved therapeutically.
For further information please contact Dr. Jürgen Alphonsus or Assoc.Prof. Mag. Dr. Stefan Tögel.
For information on the FWF-funded project P35438-B please visit the FWF project website.

As an integral part of the Department of Orthopedics and Trauma Surgery, the KCLOB is involved in a number of clinical project and trials that aim to develop novel modalities for the diagnosis or treatment of patients with musculoskeletal disorders. In such interdisciplinary projects, we take in a supportive role, contributing expertise in cell/molecular biology, histology or biomarker analysis, and providing input from a basic research perspective.
Supported studies have focused on material failure in spine surgery, genetic causes for neuropathies, biological cartilage regeneration in a large animal model, clinical trials of a personalised bone implant for the treatment of degenerative spine disorders, efficacy of intraarticular LNA043 in regenerating articular knee cartilage, and the development of novel MR imaging modalities of cartilage and meniscus.
For further information please contact Assoc.Prof. Mag. Dr. Stefan Tögel and see our publications.
